By Kyle Proffitt
October 21, 2025 | Researchers from Tohoku University, Japan have succeeded in creating room-temperature rechargeable magnesium batteries, made possible using an oxide cathode with a unique amorphous structure that facilitates Mg ion movement. The batteries produce up to 150 mAh/g discharge capacity, cycle 200 times with ~80% capacity retention, and in coin cell format were shown to power a blue LED for several minutes. The work was published last month in Communications Materials (DOI:10.1038/s43246-025-00921-0).
Magnesium (2+) Too?
Rechargeable magnesium batteries (RMBs) are yet another variant on the general operating principles of lithium-ion and sodium-ion batteries, but magnesium carries the charge, explained first author Tomoya Kawaguchi, now at Argonne National Laboratory, in email correspondence with Battery Power Online. As electrification gains ground in more and more areas, additional options are welcome—necessary even—because lithium is unlikely to shoulder the entire load. “RMBs have several intrinsic advantages: they are inherently safe and stable, can achieve high specific capacity, and are cost-effective from a resource standpoint because magnesium is abundant,” Kawaguchi said.
Like sodium, magnesium is easy to find; it is the eighth most abundant element in the earth’s crust and the 3rd most common in seawater, whereas lithium is comparatively rare and unevenly distributed. A key potential benefit with RMBs is the feasibility of using Mg metal anodes. Magnesium has an advantage in that each atom carries (and releases upon ionization) two electrons and thus twice the energy per atom relative to lithium or sodium. The gravimetric energy density outpaces sodium as a result, but lithium still wins here because it is so much lighter (Li, 6.9 g/mol; Mg, 24.3 g/mol). However, because magnesium is over three times more dense than lithium, it can produce the best numbers for volumetric energy density (Mg, 3833 mAh/cm3 vs. Li, 2061 mAh/cm3). That means that more energy, at least at the anode, can theoretically be packed into a smaller, heavier unit. Compared with lithium and sodium, magnesium is also less reactive and shows favorable even plating, which should simplify handling and safety.
Rechargeable Magnesium Batteries Face Challenges
A prototype RMB was developed back in 2000 using a sulfide cathode, an organohaloaluminate salt electrolyte, and Mg metal anode, but the main idea was to compete with low-energy-density lead-acid batteries. RMBs have since lagged in development because of difficulties in identifying compatible combinations of cathode, electrolyte, and Mg metal. Oxide cathodes are attractive for improving energy density, but they have performance issues. “Because Mg is a divalent cation, it interacts strongly with surrounding species, such as oxygen in oxide materials,” Kawaguchi explained. “As a result, the movement of Mg ions within solids is very sluggish, which has limited RMB operation to elevated temperatures and caused slow charge and discharge rates.” That same higher valency that helps boost capacity for magnesium creates a challenge for ion movement.
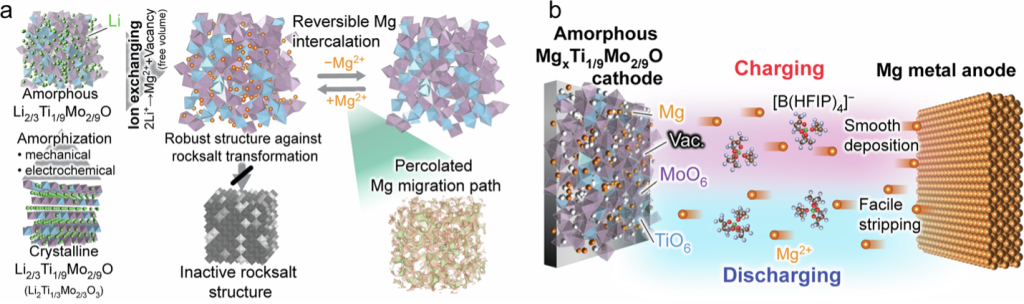
Against this backdrop, Kawaguchi and colleagues developed a cathode material that solves many of these issues. Their cathode actually starts with lithium-titanium-molybdenum oxide (Li2Ti1/3Mo2/3O3), which has a rocksalt crystalline orientation, but a ball-milling step induces a more amorphous, looser structure (Fig. 1). Then, ion exchange is performed to replace most of the lithium with magnesium, resulting in a cathode with formula Mg0.27Li0.09Ti0.11Mo0.22O (MLTMO).
This ion swap accomplishes a couple of different things. When magnesium replaces lithium, it’s not one for one, again because of the extra electron. One Mg2+ replaces two Li+, as far as charge balance in coordinating with the transition metals and oxygen goes, but this leaves behind vacancies, or gaps, where half of the lithium used to reside. That turns out to be a good thing. The vacancies give room for Mg ions to migrate, improving those typically sluggish kinetics. At the same time, the MLTMO strengthens a predominantly amorphous structure. In many spinel or layered oxides, magnesium insertion can induce transition into a rocksalt form that is electrochemically inactive. However, the amorphous structure of MLTMO is robust against this transition and can maintain a more stable structure during Mg insertion and extraction.
Full RMBs That Cycle
After preparing the MLTMO cathode, the researchers combined it with Mg[B(HFIP)4]2-triglyme electrolyte, a glass separator, and Mg foil anode and started cycling complete cells. They demonstrated a maximum discharge capacity of 150 mAh/g at 5 mA/g rate, and the cells retained 70 mAh/g at a much faster 500 mA/g, indicating that the materials are amenable to rapid cycling. After 200 cycles at 10 mA/g, the batteries retained ~80% of peak capacity, and the relatively flat retention curve past the first few formation cycles suggests many more cycles are possible. The cathode also showed ~255 Wh/g energy density (a function of voltage and capacity) at the slowest discharge rate. All of this was accomplished at room temperature.
Just creating functional full-cell RMBs was a major step, Kawaguchi said, because of incompatibilities between materials. As an example, “electrolytes compatible with oxide cathodes, such as Mg[TFSA]2, can passivate [form an ionically insulating surface on] the Mg metal anode unless additives are used, preventing discharge.” Those cells would not cycle well. Kawaguchi added that as a result, “most previous studies have focused only on either the cathode or the anode, and demonstrations of both together have been very limited.” Demonstrating that their coin cell battery could generate sufficient voltage (>2.5 V) to drive a blue LED for several minutes was further indication of significant progress relative to other RMB efforts.
Prove It
The report contains a high level of sophisticated experiments to confirm mechanistic activity in the batteries. Kawaguchi explained that with RMBs, “many previous studies have not clearly verified that Mg insertion/extraction actually occurs in the cathode structure.” He added that “RMBs often suffer from significant side reactions, so electrochemical capacity alone cannot be taken as evidence of Mg insertion/extraction.” In their study, X-ray diffraction revealed the majority amorphous structure of MLTMO, and elemental analysis of the cathode was performed before and after cycling to reveal Mg movement. This analysis also demonstrated that while Li is present in the cathode, it contributes very little to capacity (there was also no lithium salt included in the electrolyte to facilitate lithium ion transfer).
They used lithium in their cathode precursor because its behavior is well understood in lithium-ion batteries, but “other monovalent cations, such as K or Na, could in principle be used in the same ion-exchange process, and exploring those alternatives will be an important direction for future work,” Kawaguchi said. Additional X-ray absorption spectroscopy demonstrated the participation of molybdenum in redox chemistry during cycling, further indicating that capacity does not originate from side reactions but from Mg insertion and extraction. “We made a particular effort to analyze the battery operation from multiple perspectives, using chemical composition analysis, X-ray structural and spectroscopic techniques, and computational modeling, so that other researchers can clearly evaluate the reliability of our findings and use them as a foundation for further progress in RMB research,” Kawaguchi said.
Magnesium Batteries of the Future
Kawaguchi cautioned against any unrealistic expectations that commercial RMBs are right around the corner. “The field is still in its early stages, and continued scientific progress will be essential for the eventual realization of magnesium-based energy storage in society,” he said. “Every component, including the electrolyte, is still under development.” Similarly, he champions a long view that would not dismiss RMBs as potential competitors based on their current performance metrics. No, they’re not EV-ready (and may never be), but Kawaguchi pointed to the example of LFP, which initially showed lackluster performance, but over the course of many years has developed into a mainstay of Li-ion chemistry.
Right now, he said that RMBs are “particularly attractive for large-scale stationary energy storage, such as those integrated with renewable energy sources, where low-cost and sustainable materials are essential.” Additionally, a “combination of safety, simplicity, and scalability suggests that RMBs could also be applied in other areas in the future, beyond stationary storage.”
With regard to the RMBs developed here, he sees practical next steps. The capacity of the MLTMO cathode rivals that of spinel cathodes such as LMNO in lithium-ion batteries, while falling a bit short of LFP. The energy density suffers, however, because the operating voltage for their cells is much lower. Kawaguchi explained that “this is because the elements used in the present cathode, Mo and Ti, exhibit relatively low redox potentials.” Those elements were chosen for stability and compatibility with electrolyte, but “exploring other transition-metal combinations to increase the voltage is a clear next direction” that can lead to more practical competitors for current battery systems.







