Isidor Buchmann, CEO & Founder
Cadex Electronics Inc.
A good charger provides the base for well-performing and durable batteries. In a price-competitive market chargers often receive low priority. The battery and charger must go together like horse and carriage, and this is not always the case. Engineers are often not fully aware of the complex power requirement of a portable device and the need to charge under adverse conditions.

Chargers are divided into personal and industrial, “smart” and “dumb,” slow, fast and ultra-fast types. Consumer products come with a low-cost personal charger that performs well when used as directed. The industrial charger is often made by a third party and includes special features, such as charging at adverse temperatures. Although batteries operate below freezing, not all chemistries can be charged when cold and most Li-ion falls into this category. Lead and nickel-based batteries accept charge but at a lower rate.
Some Li-ion chargers include a wake-up feature, or “boost,” to allow recharging if a Li-ion battery have fallen asleep due to over-discharge. A sleep condition can occur when storing the battery in a discharged state and the self-discharge brings the voltage to the cut-off point. A regular charger treats such a battery as unserviceable and the packs are discarded. Boost applies a small charge current to raise the voltage to between 2.20 and 2.90V/cell and activate the protection circuit, at which point a normal charge commences. Caution applies if Li-ion has dwelled below 1.5V/cell for a week or longer
Lead- and lithium-based chargers operate on Constant Current Constant Voltage (CCCV) by which the voltage is capped when reaching a set limit. At this point of the charge cycle, the battery begins to saturate and the current drops. Full-charge occurs when the current drops to a set level. Lead acid requires a periodic full saturation to prevent sulfation.
Nickel-based batteries charge with constant current and the voltage is allowed to fluctuate freely. This can be compared to lifting a weight with an elastic band where the hand moves ahead of the load. Full charge detection occurs when observing a slight voltage drop after a steady rise. This method is known as the Delta Voltage Delta Temperature (DVDT) and works well with rapid and fast charge. To safeguard against anomalies, such as shorted or mismatched cells, the charger should include a plateau timer to terminate charge if no voltage delta is measured, as well as a temperature sensors.
A temperature rise is normal with nickel-based batteries, especially when reaching the 70 percent charge level. The reason for this is a decrease in charge efficiency and the charge current should be lowered to limit stress. When “ready,” the battery must cool down. If the temperature stays above ambient, then the charger is not performing right and the battery should be removed. Extended trickle charge on nickel-based batteries inflicts damage. NiCd and NiMH should not be left in the charger unattended for weeks and months. If not required, store them in a cool place and apply a charge before engagement.
Lithium-based should always stay cool on charge. Discontinue using the battery and/or charger if the battery heats up on charge. Li ion cannot absorb over-charge and therefore does not receive trickle charge when full. It is not necessary to remove Li-ion from the charger, however, if not used for a week or more, it is always best to place the pack in a cool place and recharge before use.
The most basic charger is the overnight charger, also known as slow charger. This goes back to the old nickel-cadmium days where a simple charger applied a fixed charge of about 0.1C (one-tenth of the rated capacity) as long as the battery was connected. Slow chargers have no full-charge detection; the charge stays engaged and a full charge of an empty battery takes 14–16 hours. When fully charged, the slow charger keeps NiCd lukewarm to the touch. Because of its reduced ability to absorb over-charge, NiMH should not be charged on a slow charger. Low-cost consumer chargers to charge C AA and AAA cells often use this charger method, so do some children’s toys.
The rapid charger falls between the slow and fast charger and is used in consumer products. The charge time of an empty pack is 3–6. When full, the charger switches to “ready.” Most rapid chargers include temperature sensing to safely charge a faulty battery.
The fast charger offers several advantages and the obvious one is shorter charge times. Short charge times demand tighter communication between the charger and battery. At a charge rate of 1C, which the fast charger typically uses, an empty NiCd and NiMH charges in a little more than an hour. As the battery approaches full charge, some nickel-based chargers reduce the current to adjust to the lower charge acceptance. The fully charged battery switches to trickle charge, also known as maintenance charge. Most of today’s nickel-based chargers have a reduced trickle charge to also accommodate NiMH.
Li-ion charges are most efficient and charge the battery to 70 percent in less than an hour. The extra time is devoted for the long saturation charge that is not mandatory as it is for lead acid. In fact, it is better not to fully charge Li-ion as it will last longer. Of all chargers, the Li-ion charger is the most simplistic. No trickery applies to improve battery performance and longevity. Only the CCCV method works.
Lead acid cannot be fast-charged and the term “fast-charge” is a misnomer. Most lead acid chargers charge the battery in 14 to 16 hours; anything slower is a compromise. Lead acid can be charged to 70 percent in about eight hours; the all-important saturation charge takes up the remaining time. A partial charge is fine provided the lead acid occasionally receives a fully saturated charge to prevent sulfation.
Ultra-fast Charger
Nowhere is ultra-fast charging in bigger demand than with the electric vehicle. Recharging an EV in minutes replicates the convenience of filling 50 liters (13 gallons) of fuel into a tank that delivers 600kWh of energy. Such large energy storage in an electrochemical device is not practical as a battery with this capacity would weigh six tons. Current Li-ion only produces about 150Wh per kg; the energy from fossil fuel is roughly 100 times higher.
Charging an EV will always take longer than filling a tank, and the battery will always deliver less energy per weight than fossil fuel. Breaking the-rule-of law and forcing ultra-fast charging adds stress, even if the battery is designed for such purpose. We must keep in mind that a battery is sluggish in nature and like an aging man; its physical conditions become less ideal with use. So is the ability to fast-charge.
Whether it’s an EV, e-bike, a flying object, a portable device or a hobby gadget, the following conditions must be respected when ultra-fast charging a battery:
• The battery must be designed to accept an ultra-fast charge and must be in good condition.
• Ultra-fast charging only applies during the first charge phase. The charge current should be lowered after the battery reaches 70 percent state-of-charge (SoC).
• All cells in the pack must be balanced and have ultra-low resistance. Aging cells often diverge in capacity and resistance, causing mismatch and undue stress on weaker cells.
• Ultra-fast charging can only be done under moderate temperatures. Low temperature slows the chemical reaction. Unused energy turns into gassing, metal-plating and heat.

powerful machinery is easy to build, but it’s the tracks that limit the speed.
An ultra-fast charger can be compared to a high-speed train traveling at 300km per hour (188 mph). Increasing power is relatively simple but it’s the tracks that govern the permissible speed of a train and not the machinery. In the same manner, the condition of the battery dictates the charging speed. A well-designed ultra-fast charger should include temperature compensations and other safety features that lower the charge current when certain conditions exist and halt the charge if the battery is put under undue stress.
A “smart battery” running on SMBus or other protocols instructs the charger to provide the maximum allowable charge current based on the battery system. What most “smart” systems ignore is the condition of the battery. They assume that packs are running close to the original capacity and that all cells are well balanced. Such a condition only exists when the battery is new. An ultra-fast charger should evaluate the condition of “chemical battery” and make adjustments if needed instead relaying fully on the orders given by the “digital battery.”
The maximum charge current a Li-ion can accept is governed by cell design, and not the cathode material as is commonly assumed. The goal is to avoid lithium-plating on the anode and to keep the temperature under control. A thin anode with high porosity and small graphite particles enables ultra-fast-charging because of the large surface area. Although these so-called Power Cells can be charged and discharged at high currents, the energy density is low. The Energy Cell, in comparison, has a thicker anode and lower porosity but this battery should be charged at less than1C. Some hybrid Cells in NCA (nickel-cobalt-aluminum) can be charged at 4C with moderate stress.
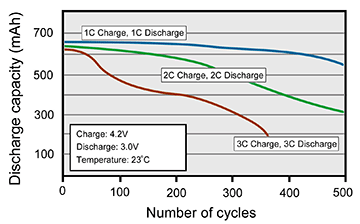
When possible, apply the ultra-fast charge only when necessary. A properly designed ultra-fast charger should allow charge-time selection to give the user the option to choose the least stressful charge for the time allotted. Figure 3 compares the cycle life of a typical lithium-ion battery when charged and discharged at 1C, 2C and 3C rates.
Summary
All batteries perform best at room temperatures with a gentle charge and discharge. However, such a sheltered life style does not always apply to real world situations requiring a compact pack that must be charged quickly and deliver a heavy load. Such batteries can by deployed safely but expect a short life expectancy. Typical applications are drones, EV races and hobbyist contests.
If fast charging and high load requirements are prerequisites, the rugged Power Cell is ideal but this increases battery size and weight. An analogy is choosing a heavy diesel engine to run a large truck instead of a souped-up engine designed for a sports car. The big diesel will outlive the light engine even if they have the same horsepower. Going heavier will at the end be more economical.
Simple Guidelines on Chargers
• Use the correct charger for the intended battery chemistry. Most chargers only serve one chemistry.
• Recommend charging at a moderate rate. Ultra-fast charging causes undue stress even if the battery can take it.
• Do not apply fast and fast charge when the battery is cold or hot. Charge batteries at moderate temperatures.
• Do not apply fast and ultra-fast charge to aged and low-performing batteries. Very few chargers are able to assess the condition of a battery and govern the fast-charge accordingly.
• Observe battery temperature when using a low-cost charger. Remove battery when warm.
Isidor Buchmann is the founder and CEO of Cadex Electronics Inc. For three decades, Buchmann has studied the behavior of rechargeable batteries in practical, everyday applications, has written award-winning articles including the best-selling book “Batteries in a Portable World,” now in its third edition. Cadex specializes in the design and manufacturing of battery chargers, analyzers and monitoring devices. For more information on batteries, visit www.batteryuniversity.com; product information is on www.cadex.com.







